In Oncology R&D, we develop pioneering new immuno-oncology therapeutics based on our proprietary , multi-specific antibody technology.
Our Platform Technologies
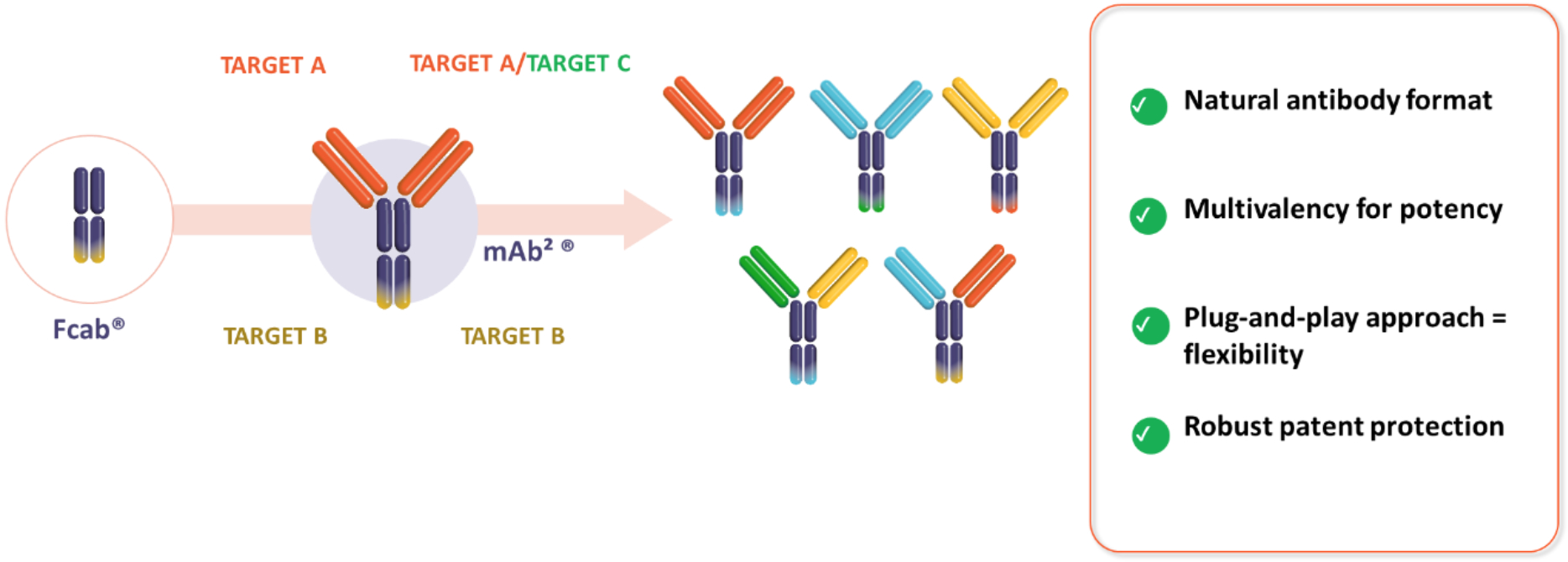
Our technology uses the Fcab® platform, a “plug and play” clinical stage antibody domain developed by F-star Therapeutics and acquired by invoX in 2023.
Our mAb²® bispecific antibody technology platform enables rapid discovery of differentiated drug product candidates and has been used to generate a clinical pipeline of tetravalent mAb²® bispecific antibodies with a natural human antibody format providing advantages such as a straightforward manufacturing process, favourable safety profile and strong biological potency.
Our Clinical Programmes
Our clinical stage multispecific antibody platform has delivered a proprietary and partnered pipeline of promising clinical and pre-clinical drug product candidates. Our pipeline includes clinical stage mAb²® bispecific antibody programmes focused on next-generation immuno-oncology. These tetravalent mAb²® bispecific molecules use our versatile, next-generation technology to generate a single molecule to target multiple complementary immune mechanisms at once with differentiated mode of action.