In Oncology R&D, we develop pioneering new immuno-oncology therapeutics based on our proprietary , multi-specific antibody technology. Our Oncology R&D Team is comprised of invoX Therapeutic Innovation (iTI) and our Development function, which includes Translational Science capabilities and our Clinical Development team.
Our Platform Technologies
The invoX Therapeutic Innovation (iTI) team is a discovery and preclinical research team based in Cambridge, UK with a track record in antibody discovery and moving innovative programmes into clinical development. Our aim is to create new pipeline opportunities using next generation antibody platforms. A core feature of these platforms is the Fcab® platform, a “plug and play” clinical stage antibody domain developed by F-star Therapeutics and acquired by invoX in 2023.
Our mAb²® bispecific antibody technology platform enables rapid discovery of differentiated drug product candidates and has been used to generate a clinical pipeline of tetravalent mAb²® bispecific antibodies with a natural human antibody format providing advantages such as a straightforward manufacturing process, favourable safety profile and strong biological potency.
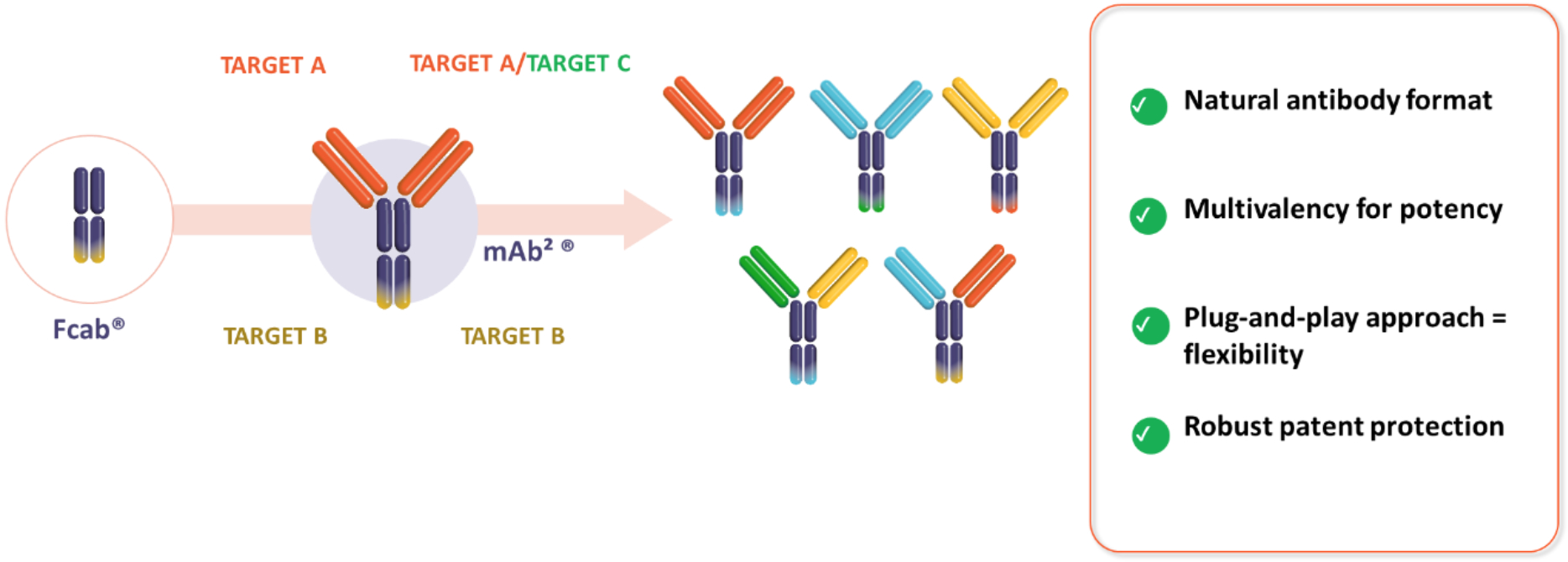
Furthermore, we have built deep knowledge and expertise in immune co-stimulatory pathways and have demonstrated that our antibody platform has the potential to generate best-in-class agonists. Our current focus continues to progress preclinical stage mAb²® bispecific antibodies and to create technology platforms based on next-generation antibody formats such as trispecific antibodies and antibody-drug conjugates (ADCs).
Our Clinical Programmes
Our clinical stage multispecific antibody platform has delivered a proprietary and partnered pipeline of promising clinical and pre-clinical drug product candidates. Our clinical pipeline includes three-clinical stage mAb²® bispecific antibody programmes focused on next-generation immuno-oncology: FS222, FS120 and FS118. These tetravalent mAb²® bispecific molecules use our versatile, next-generation technology to generate a single molecule to target multiple complementary immune mechanisms at once with differentiated mode of action.
Our Translational Sciences team has an established record of transitioning oncology programmes into clinical development and providing data to enable clinical decision making. With expertise in biology, bioanalytics and biomarkers, data science and ‘omics, and non-clinical safety, the group helps our programmes gain a deep understanding of drug mechanism of action, establish optimal dosing strategies, and identify patients most likely to respond to treatment. To achieve this, the Translational Sciences team works closely with external experts, through strategic partnerships and collaborations.
mRNA Research
We have also invested in pHion Therapeutics, a UK-based mRNA delivery company. pHion is developing a pipeline of therapeutic and prophylactic vaccines focussed on oncology and infectious disease. The company’s proprietary RALA platform can deliver anionic molecules such as mRNA and saRNA in a stealth-like way, evading detection, to generate a potent antigen-specific CD8+ T-cell response. The RALA peptide-based drug delivery system is based on a sequence of 30 amino acids which condenses anionic cargo into nanoparticles that are highly efficient at cellular entry with the potential to outperform currently available technologies. Compared with traditional mRNA vaccine delivery approaches, the RALA platform has the added advantage of ease of scalability and logistics, including stability at room temperature. pHion’s lead programme, PTX_V1, is a first-in-class therapeutic vaccine in late preclinical development for the treatment of human papilloma virus-related cancers.